Updates on GCP Guidelines
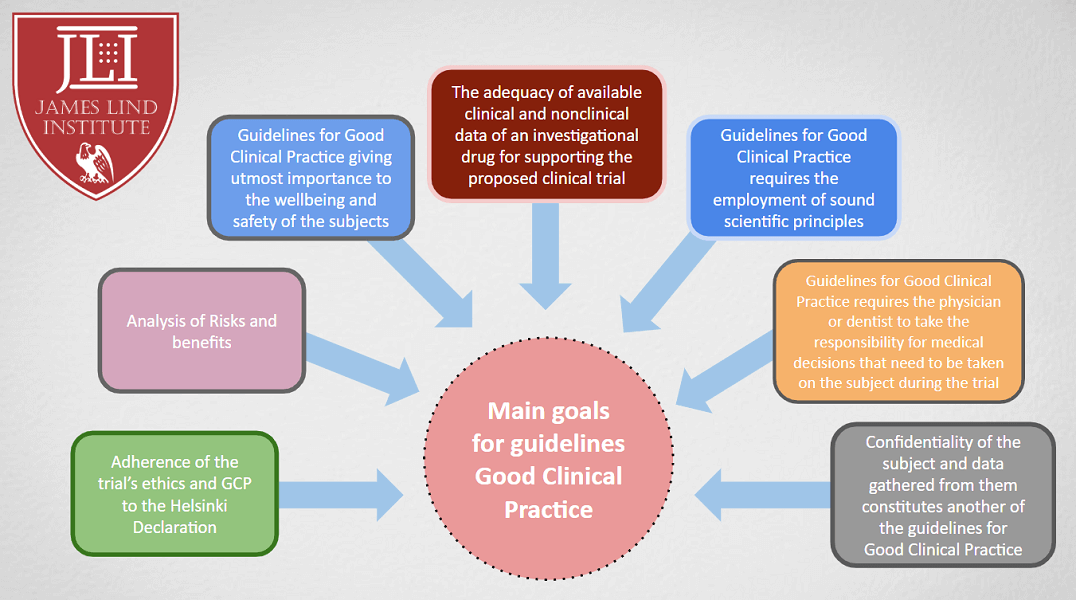
Posted onClinical research is necessary to establish the efficacy and safety of specific health products and medical practices. All the key changes, revisions and amendments in guidelines are designed to help clinical researchers and practitioners to protect human subjects and properly document trial results, maintain data quality and integrity. Good Clinical Practice (GCP) Guidelines Clinical research […]