MedDRA, the Medical Dictionary for Regulatory Activities, is a medical terminology used to classify adverse event information associated with the use of biopharmaceuticals and other medical products. (Defined by MedDRA MSSO).
Big-Pharma used one or more available sources for medical terminology coding, like COSTART, WHO-ART, WHO-DRUG, etc., for many years. But in order to bring consistency and uniformity to the coded data being submitted to it, the FDA has recently committed to a standard coding dictionary namely, MedDRA. The ICH also mandates the use of MedDRA for safety reporting in clinical trials.
Most of the clinical trials are multi-centred and are being conducted in different geographies. To analyse the clinical data and to prepare the clinical study report using the data collected form different geographies, the collected data should be in a single format to avoid discrepancies. To avoid such discrepancies, coding of the medical terminology into a single universally accepted format is necessary. This can be achieved by using different medical dictionaries as mentioned above. But, FDA and ICH has mandated the use of MedDRA for medical terminology coding in clinical trials.
The Maintenance and Support Services Organization (MSSO), an organization that reports to the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA), manages MedDRA. The current version of MedDRA is version 14.1, which was released in September 2011. MedDRA version updates are released twice a year (March and September). The MSSO serves as the repository, maintainer, and distributor of MedDRA as well as the source for the most up-to-date information regarding MedDRA and its application within the biopharmaceutical industry and regulators.
MedDRA was classified into five categories from the broadest grouping found in System Organ Classes (SOCs) to the maximum specificity found in Lower Level Term (LLTs). The hierarchy of MedDRA is represented as follows:
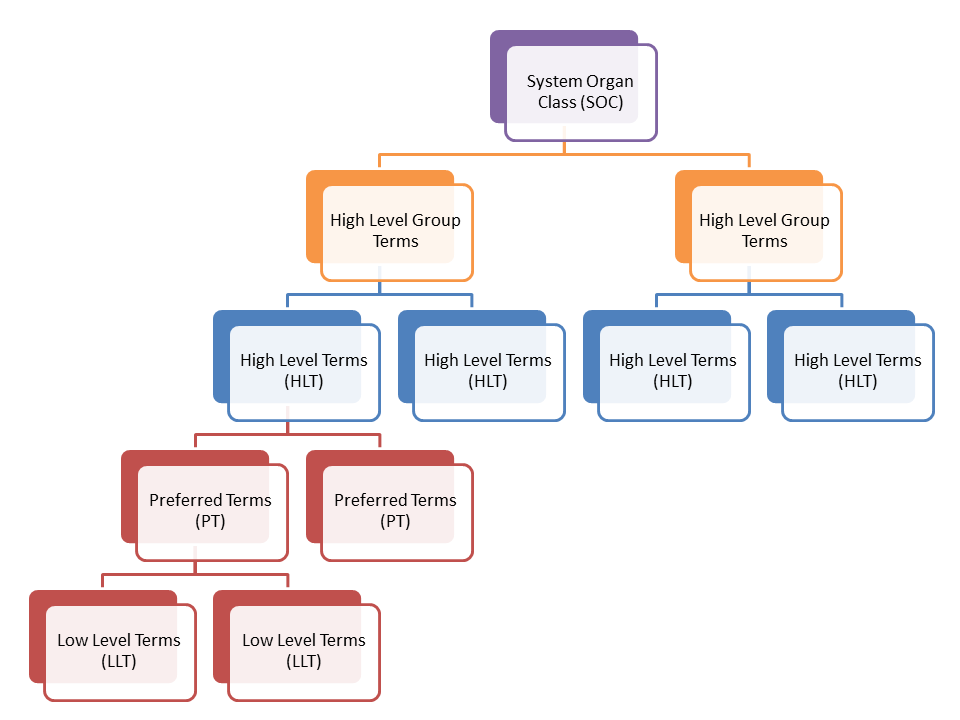
The MedDRA is integrated with the clinical trial management software to enhance the autoencoding of the medical terminology within the system. Autoencoding systems typically use verbatim matching methods whereby a computer program attempts to assign a dictionary term (code) to the raw adverse event term based on matched spellings.
MSSO offers a desktop version of MedDRA for medical terminology coding. It is a beta version and can be downloaded from the MSSO website.
(http://www.meddramsso.com/subscriber_download_tools_browser.asp)

