Pre-clinical drug development process is a risk based process that involves safety and efficacy evaluation of drugs in animal species that extrapolate to potential human outcome. The pre-clinical pharmacologic and toxicological drug responses with respect to dose regimen and route of administration enable to initiate and continue research in human beings. In general, pre-clinical studies are performed to predict the safety and efficacy data from the animal models which support the conduct of research in human beings.
Before a new active substance can be used as a medicinal product, it has to be tested for its safety and efficacy in animals prior to its use in humans. This testing in animals is termed as pre-clinical studies. The pre-clinical studies also have to get approval by regulatory authorities. The regulatory authorities must ensure that trials are conducted in ethical ad safe manner and should give approval for those drugs which are found to be safe and effective. ICH has established a basic set of guidelines that outline the technical requirements of acceptable preclinical drug development.
The pre-clinical drug development process can be performed in two disciplines: General Pharmacology and Toxicology…
Pharmacology deals with the pharmacokinetic and pharmacodynamic properties of drug. It is important to investigate undesirable pharmacological activity in appropriate animal models and monitoring them in toxicological studies. Pharmacokinetic studies are very important to reveal the safety and efficacy parameters in terms of absorption, distribution, metabolism and excretion (ADME). ADME studies or Pharmacokinetic studies determine the drug pathway in the body. These studies give data on absorption rate for different routes of administration, which helps in dosage form selection, distribution mechanism, rate of metabolism and excretion; which determines the half-life of the drug. Half-life (T1/2) of the drug explains the safety profile of the drug which is the mandatory for a drug to get approved by regulatory agencies.
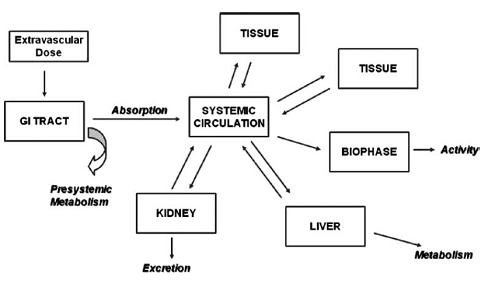
The drug distribution mechanism explains the therapeutic efficiency of the drug as it depends on the drugs availability and its ability to access its site of action. Drug metabolism gives the probability of formation of potentially toxic metabolites through biotransformation process. It also helps in understanding the metabolism pathway and enzymes involved in it.
Toxicological activity of the products can be determined using in-vitro and in-vivo assay methods which estimate the clinical relatedness of the toxicological effects of the drug. In-vitro studies can be performed to examine the direct effects on cellular phenotype and proliferation. In-vivo studies can be performed for qualitative and quantitative determination of toxicological effects. As many pharmaceutical compounds are species specific, it is important to select relevant animal species for toxicity testing. In-vivo studies to assess pharmacological and toxicological activities, including defining mechanism(s) of action, are often used to support the rationale of the proposed use of the product in clinical studies.
The following table explains the duration, time and supported clinical study phase for the preclinical studies:
[table id=1 /]
Every new drug molecule must cross the barrier of pre-clinical phase to enter into clinical trial phases. A drug that successfully completes this phase only has a 20% chance to make it to market. Pre-clinical studies limit the risk to patients to the minimum. It is mandatory to harmonize all aspects of drug according to the ethical principles of medicine and animal protection.

